Abstract
Introduction:
The search for potential new targets to improve outcomes in patients with CML is ongoing, with a view to improve treatment efficacy for those with refractory disease and increase the proportion of those eligible to attempt TKI discontinuation. CML is recognised as a particularly immune sensitive tumour and as such immune checkpoint inhibitors, which can enhance inherent immune surveillance mechanisms are an attractive proposition.
Methods:
We performed flow cytometric analysis of peripheral blood mononuclear cells for expression of PD1, CTLA-4, TIM-3 and LAG-3 on T effectors and regulatory T cells. T effectors included CD4+ and CD8+ subsets and a gating strategy of CD4+/CD25+/CD127 lo/FOXP3+ cells for Tregs was employed, whilst FOXP3 hi/CD45RA-ve cells denoted effector Tregs. FMO controls were used to determine positive populations for each immune checkpoint molecule under investigation.
Results:
Samples from 22 patients were analysed, including samples from two different time points in 4 patients. This included patients at diagnosis (n=8), those with refractory disease defined as <CCyR (n=3) and those with a response of MMR (n=6) and MR4 or greater (n=9). Patients with a response of MMR or greater were considered to have low disease burden.
Patients at diagnosis had higher Tregs as proportion of total CD4+ cells compared to those with low disease burden with a mean of 6.3 vs 4.6 (p=0.048). Similarly, effector Tregs, the most functionally suppressive Treg subset, were also higher at diagnosis at 10.3 vs 5.4 (p=0.05). No differences were observed in frequency of Tregs between refractory and low disease burden groups.
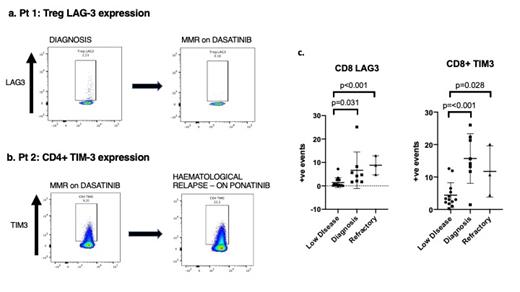
PD1 expression was higher at diagnosis in CD4+, CD8+ cells and Tregs when compared to those with low disease burden (4.75 vs 2.75, 5.85 vs 2.43 and 5.2 vs 2.78, p=0.034/p=0.003/p=0.002). Similarly, TIM3 expression was significantly higher at diagnosis compared to at low disease burden; in CD4+ at 6 vs 1.9, CD8+ at 15.71 vs 4.41 and Tregs at 5.15 vs 1.88 (p=0.027/p=<0.001/p=0.002). LAG3 expression was higher at diagnosis in CD8+ 6.67 vs 1.4 and Tregs 2.13 vs 0.68 (p=0.031/p=0.025). No significant differences were observed in CTLA4 expression between these groups although a trend towards significance was observed in Tregs with 2.36 vs 0.67 (p=0.055).
Despite low sample size, PD1 expression was also significantly higher in refractory patients compared to those with low disease burden, in CD4+ cells at 5.34, and Tregs at 5.6 (p=0.031/p=0.026). TIM3 expression was higher in CD8+ subset only at 11.74 (p=0.028). LAG3 showed higher expression in CD4+ cells at 1.56 vs 0.45 and in CD8+ cells at 8.81 (p=0.004/P=<0.001). Finally, CTLA-4 also showed higher expression in CD4+ cells at 1.59 vs 0.47 and Tregs at 1.99 vs 0.67 (p=0.026/p=0.032).
One patient was analysed at diagnosis and then again after achieving MMR following dasatinib treatment for 11 months. A significant trend of downregulation of immune checkpoint expression with successful TKI treatment was observed. For example, Treg expression of PD1 decreased from 7.03 to 4.86, TIM3 decreased from 2.57 to 0.83 and LAG3 decreased from 2.23 to 0.16 (Figure 1a), with CTLA4 remaining at a similar level. Similarly, another patient was analysed whilst in MMR and then again following haematologic relapse, one week after regaining CHR with ponatinib. In contrast, this patient showed evidence of upregulation of checkpoint molecules, particularly in TIM3 expression increasing from 9.2 to 22.2 in CD4+ cells and from 12.6 to 20.1 in CD8+ cells (Figure 1b).
Discussion:
We have shown through an extended analysis of immune checkpoint expression on lymphocytes from blood samples of patients with CML, that expression correlates strongly with leukaemia disease burden. We provide the first report, to our knowledge, to describe the increased expression of TIM3 and LAG3 in peripheral blood lymphocytes, which is of significance given the recent development of inhibitors of these molecules.
These data support the potential future use of immune checkpoint inhibitors in certain patients with high-risk disease at diagnosis as well as addition in those with inadequate response, alongside conventional TKI therapy. Moreover, these data provide a potential basis for the use of combination immune checkpoint blockade which has proven highly efficacious in other settings. Further laboratory and clinical studies evaluating these agents in CML are warranted.
Harrington: Bristol Myers Squibb: Research Funding; Incyte: Honoraria. Dillon: Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Other: Educational Events , Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Session chair (paid to institution), Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: educational events; Jazz: Other: Education events; Amgen: Other: Research support (paid to institution); Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Educational Events. Radia: Cogent Biosciences Incorporated: Other: Study Steering Committee; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Study steering group member, Research Funding; EXPLORER and PATHFINDER studies: Other: Member of the Response Adjudication Committee; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Education events. Kordasti: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Alexion: Honoraria; Beckman Coulter: Honoraria. Harrison: Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Incyte Corporation: Speakers Bureau; Sierra Oncology: Honoraria; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. de Lavallade: Incyte: Honoraria, Research Funding; Novartis: Speakers Bureau; Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal